Global In-vivo CRO Industry: Key Statistics and Insights in 2025-2033
Summary:
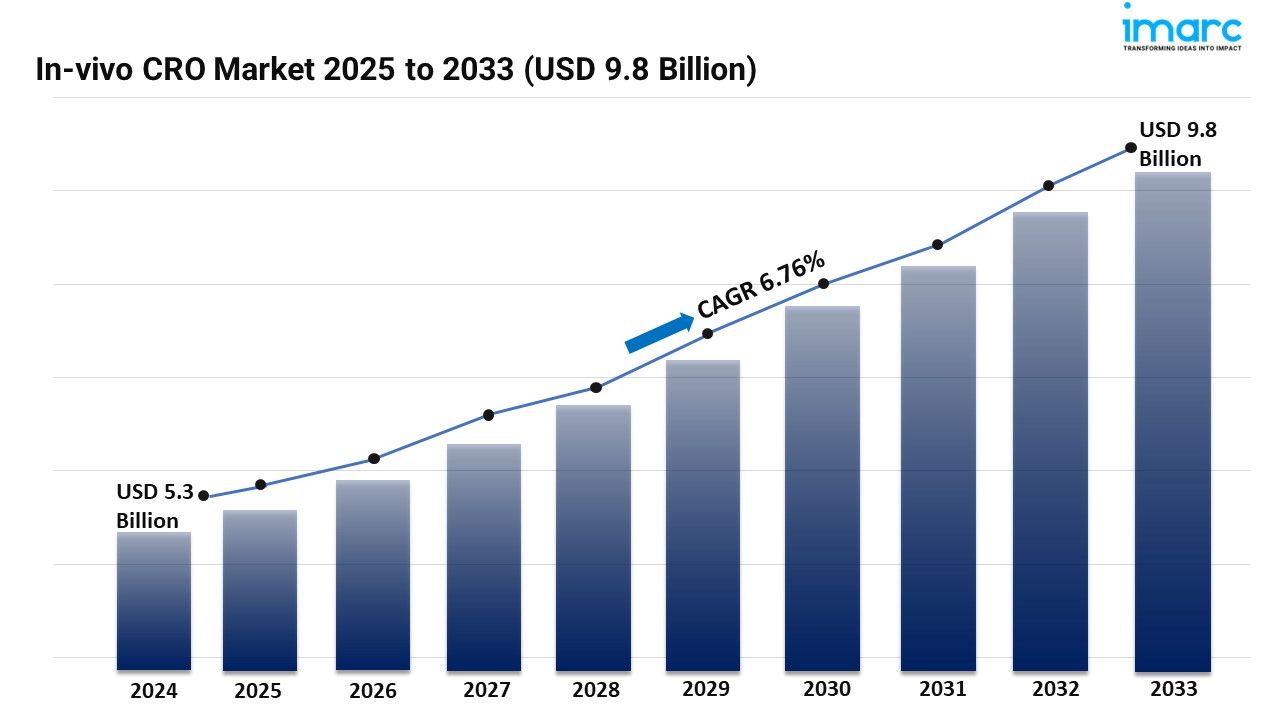
- The global in-vivo CRO market size reached USD 5.3 Billion in 2024.
- The market is expected to reach USD 9.8 Billion by 2033, exhibiting a growth rate (CAGR) of 6.76% during 2025-2033.
- North America leads the market, accounting for the largest in-vivo CRO market share.
- Rodent accounts for the majority of the market share in the type segment due to its relatively short lifespans and rapid reproduction rates.
- GLP toxicology holds the largest share in the in-vivo CRO industry.
- Oncology remains a dominant segment in the market owing to the growing number of oncology drug candidates entering clinical trials.
- The rising cost for drug development is a primary driver of the in-vivo CRO market.
- The growing preclinical research activities is reshaping the in-vivo CRO market.
Industry Trends and Drivers:
- Rising drug development costs:
The development of drugs has become complex because of the enhancement of science and technology. Thus, modern therapeutics such as biologics and personalized medicines need in-vivo, which could be expensive. Consequently, many pharmaceutical companies subcontract such challenging tasks to other competent CROs with sufficient experience and facilities. The pharmaceutical industry is always under pressure to reduce expenses towards drug development but at the same time ensuring that it has met the necessary quality and compliance measures. Systematic reviewing and analysis in-vivo studies contracted out to CROs means that there are few overhead expenses like those required for keeping facilities as well as hiring a team hence controlling costs becomes easier.
- Growing preclinical research activities:
Thus, in the context of biotechnology and personalized medicine where drug candidates are steadily proliferating, there has been a rising need for in-vivo preclinical procedures. He also established that pharmaceutical and biotech companies are engaging in significant levels of early drug discovery research, implying more in-vivo outsourcing to CROs. As existent drug development becomes more difficult and fraught and as novelties like biologics, gene therapies, and advanced therapeutics gain prominence, there is a need for a higher level of preclinical testing that encompasses in-vivo testing. CROs have developed the knowledge and experience for executing these sophisticated trials and thus become ideal partners for companies seeking to confirm their therapeutic entities.
- Technological advancements:
From here enhanced visualisation strategies provide for better tracking of the physiological and pathological events in live models. This leads to improved data on drug effectiveness and safety of the same. The combination of genomic and biomarker techniques provides for a more precise selection of appropriate models and the prediction of drug efficacy in-vivo improves the validity of associated studies. Advance in in-vivo model construction, like genetically modified animals and humanized animals, bring more accurate results in human drug sightings. Automation would help to simplify dosing as well as monitoring in the laboratory because human errors are eliminated as much as possible in the course of the study.
Request for a sample copy of this report: https://www.imarcgroup.com/in-vivo-cro-market/requestsample
In-vivo CRO Market Report Segmentation:
Breakup By Type:
- Rodent
- Rats
- Mice
- Others
- Non-Rodent
Rodent represents the largest segment due to its cost-effectiveness, well-characterized genetics, and the extensive availability of strain-specific data.
Breakup By GLP Type:

- Non-GLP
- In House
- Outsourcing
- GLP Toxicology
- In House
- Outsourcing
GLP toxicology accounts for the majority of the market share. GLP Toxicology studies are critical for regulatory submissions, necessitating comprehensive safety assessments of drug candidates, which drives their prevalence and dominance in the in-vivo CRO market.
Breakup By Indication:
- Autoimmune/Inflammation Conditions
- Rheumatoid Arthritis
- Multiple Sclerosis
- Osteoarthritis
- Irritable Bowel Syndrome
- Others
- Pain Management
- Chronic Pain
- Acute Pain
- Oncology
- Blood Cancer
- Solid Tumor
- Others
- CNS Conditions
- Epilepsy
- Parkinson’s Disease
- Huntington’s Disease
- Stroke
- Traumatic Brain Injury
- ALS
- Muscle Regeneration
- Others
- Diabetes
- Obesity
- Others
Oncology exhibits a clear dominance in the market owing to the rising prevalence of cancer and the increasing number of oncology drug candidates in development lead to a high demand for in-vivo studies focused on tumor models and therapeutic efficacy.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America enjoys the leading position in the in-vivo CRO market. North America is home to a large number of pharmaceutical and biotechnology companies, significant research funding, and a robust regulatory framework.
Top In-vivo CRO Market Leaders:
The in-vivo CRO market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies. Some of the key players in the market are:

- Charles River Laboratories International Inc.
- Evotec SE
- ICON plc
- Iris Pharma (Abionyx Pharma)
- Labcorp Drug Development (Laboratory Corporation of America Holdings)
- North American Science Associates LLC
- Parexel International Corporation
- Pharmaceutical Product Development Inc. (Thermo Fisher Scientific Inc.)
- Pronexus Analytical AB
- Syneos Health
- WuXi AppTec.
Note: If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145